Optimize Your Processes with CIPA's Smart Production Workflows
Why Implement Production Workflows with CIPA?
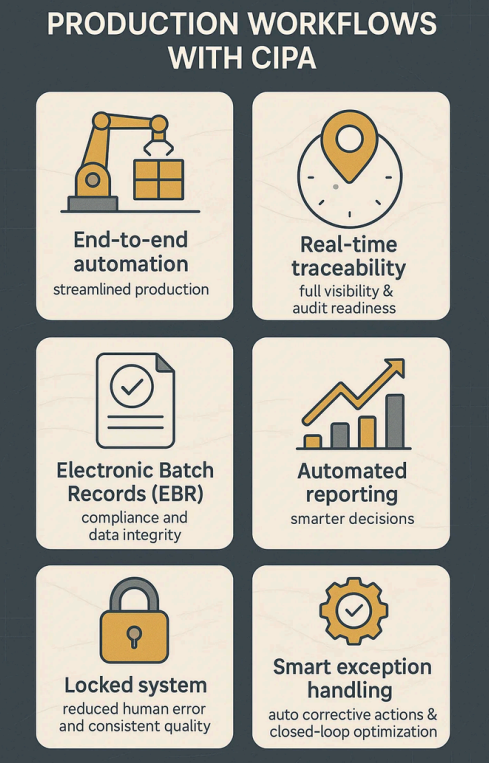
CIPA transforms production management with end-to-end automation that streamlines every production step while eliminating bottlenecks. The platform provides real-time traceability for complete visibility of all actions and parameters, ensuring audit readiness and data-driven decisions. Through Electronic Batch Records (EBR), it enforces regulatory compliance while maintaining data integrity and automated reporting. Its locked system architecture prevents human errors to guarantee consistent quality, while smart exception management automatically triggers corrective actions for deviations creating a closed-loop system that optimizes efficiency, compliance, and quality across operations.
What We Offer
Key Features of CIPA’s
🛠️Workflow Module🛠️
📌Flexible Workflow Modeling📌
-
Create custom workflows tailored to your needs.
-
Define steps, responsibilities, and deadlines.
-
Integrate validation levels (Control, Verification, Validation, Approval, Release).
📢Process Automation📢
-
Automatic workflow triggering based on events (non-conformities, audits, incidents).
-
Automatic task assignment to qualified personnel.
-
Automatic generation of reports and alerts for bottlenecks.
🔍 Real-Time Tracking and Notifications🔍
-
Dynamic dashboards to visualize workflow progress.
-
Alerts and reminders to prevent delays.
-
Real-time edits and updates as processes evolve.
🔗Integration with Other CIPA Modules🔗
- 🚀 With “Anomalies” → Trigger corrective workflows for non-conformities.
- 🔍 With “Actions” → Track action plans in a structured flow.
- 📋 With “Audits & Inspections” → Standardize audit tracking and deviation management.
- ⚠️ With “Problems & Events” → Automate industrial incident management.
Added Value for Your Company !
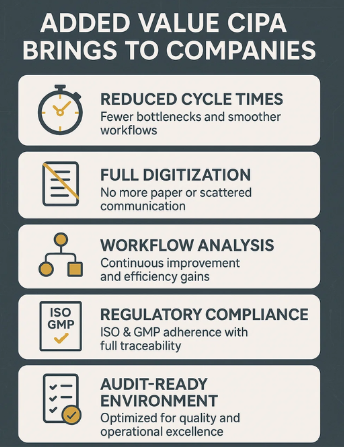
CIPA drives operational excellence by reducing cycle times to minimize bottlenecks and ensure smoother workflows, while full process digitization eliminates paper-based validations and scattered communications. The platform enables continuous improvement through workflow analysis to uncover optimization opportunities, and ensures compliance with ISO & GMP standards, meeting all regulatory requirements with complete traceability. Together, these capabilities create a streamlined, audit-ready production environment built for efficiency and quality.
Testimonials and Use Cases
Frequently Asked Questions
What is Electronic Batch Record (EBR), and why is it important?
EBR is an electronic record of all steps in a production batch, ensuring compliance, data integrity, and simplified audits.
Can certain steps in a production workflow be locked?
Yes, CIPA allows locking production steps to ensure all required validations are completed before moving to the next phase.
How does CIPA track production deviations?
Through workflow integration with anomalies and corrective actions, CIPA lets you quickly track and resolve any production deviations.
Switch to Digitized Workflows with CIPA
Ready to test the solution?
Try CIPA for free for 14 days
Ready to improve your production workflows?
Schedule a demo with our team.
