Anomaly Management with CIPA
Digitize Your Quality and Compliance Processes
Anomaly Management with CIPA
Improve non-conformity management for quality & compliance🚀
In the manufacturing, pharmaceutical, and food industries, effective anomaly management is essential to ensure product quality, process safety, and compliance with regulations (ISO, GMP, IFS, GMP, FDA, etc.).
With CIPA, simplify the detection, recording, analysis, and resolution of anomalies in real-time to ensure rigorous tracking and improve your operational performance.
Why digitize anomaly management with CIPA? 🔍
70% of industrial companies face challenges in managing anomalies and non-conformities. CIPA provides a comprehensive digital solution to:
✅ React quickly to anomalies with real-time alerts.
✅ Reduce the rate of non-conformities through centralized and standardized management.
✅ Improve traceability and auditability of corrective and preventive actions (CAPA).
✅ Automate data collection and analysis to optimize decision-making.
✅ Ensure regulatory compliance by providing precise and structured tracking.
📊 Result? More effective anomaly management, better compliance with standards, and reduced costs related to quality defects.
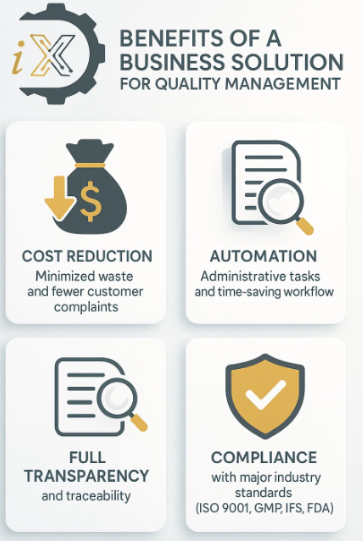
Added Value for Your Business
By implementing our solution, businesses can significantly reduce quality costs through minimized waste and fewer customer complaints.
The automation of administrative tasks and streamlined tracking processes lead to substantial time savings. Additionally, the system ensures total transparency and traceability, enabling management to remain fully compliant with industry regulations. This includes adherence to key standards such as ISO 9001, GMP, IFS, and FDA, thereby enhancing overall compliance and operational efficiency.
What We Offer
Key Features of CIPA for Anomaly
🛠️Management🛠️
📌 Real-time anomaly detection and recording📌
- Intuitive interface accessible via PC, tablet, and mobile.
- Add photos, videos, and documents for better documentation.
- Simplified reporting through predefined fields based on quality standards.
📊 Automated classification and prioritization📊
-
Categorization of anomalies by:
🔹 Type (Quality, Safety, Maintenance, Production, Logistics, etc.).
🔹 Severity (Minor, Major, Critical).
🔹 Impacted department (Production, QC, R&D, Maintenance, etc.). -
Automatic assignment to relevant teams with optimized intervention deadlines.
📍 Complete tracking, traceability, and auditability📍
-
Management of the anomaly lifecycle:
✅ Reporting → Investigation → Corrective/Preventive Action (CAPA) → Validation → Closure. -
Automatic recording of interactions to ensure flawless auditability.
-
Modification history and automatic generation of audit reports.
🔔Smart notifications and alerts🔔
-
Alerts in case of intervention deadline overruns.
-
Notifications to quality managers and field teams.
-
Reminders for pending CAPA and corrective actions.
📈 Advanced analysis and reporting 📈
-
Interactive dashboards with key performance indicators (KPIs): anomaly rate, processing times, recurring trends.
-
Automatic generation of quality reports for audits and follow-up meetings.
-
Data export to Excel, PDF, and integration with BI tools.
Accelerate Your Industrial Digital Transformation
CIPA is the digital solution for effective anomaly management and compliance with standards like ISO 9001, GMP, IFS, and FDA. By targeting keywords such as anomaly management, CAPA software, and quality digitization, it aims to attract quality, production, and maintenance managers while improving search engine visibility.
The goal is to generate qualified leads and support industrial continuous improvement through streamlined operations and regulatory adherence.
Frequently Asked Questions
Does CIPA manage corrective and preventive actions (CAPA)?
Yes, CIPA includes comprehensive CAPA management with detailed and automated tracking.
Can anomaly reports and data be exported?
Yes, all reports can be exported to Excel, PDF, and integrated with your BI tools.
Is CIPA compliant with quality standards?
Yes, CIPA meets the requirements of ISO 9001, GMP, IFS, GMP, FDA and other sector-specific regulations.
Switch to Digital Anomaly Management with CIPA
Ready to test the solution?
Try CIPA for free for 14 days
Want to improve your anomaly management?
Schedule a demo with our team.
